What is Tungsten [Applications, Properties, Types, Distribution]

Tungsten is a metallic element with the symbol (W) and atomic number 74, belonging to the VIB family of the sixth cycle in the periodic table. Tungsten is mainly a hexavalent cation in nature, with an ion radius of 0.68 × 10⁻¹ºm. Due to the small radius of W6+ions, high electricity price, strong polarization ability, and easy formation of complex anions, tungsten is mainly in the form of complex anions [WO₄]²⁻, which combine with cations such as Fe²⁺, Mn²⁺, Ca²⁺in the solution to form precipitates of wolframite or scheelite.
Introduction
Tungsten Uses and Applications
Tungsten has characteristics such as high melting point, high density, high hardness, high strength, and good corrosion resistance, making it widely used and applied in various fields.

- Burner and Rocket Engine: Tungsten is a thermally stable material with extremely high melting point and resistance to thermal corrosion, which can resist oxidation and corrosion at high temperatures. Widely used in high-temperature environments.
- Electronics and Power: Tungsten has good conductivity, low coefficient of expansion, and high melting point, making it widely used in the electronics and power industries. Tungsten electrodes are used for arc welding, arc cutting, high-temperature resistant electronic electrodes, and trigger electronic devices.
- Alloy Material: Tungsten can form high-strength alloy materials with other metals and has good practical performance. Tungsten alloys are used in the production of high-speed cutting tools, high-temperature alloys, steel smelting tools, aerospace components, etc.
- Bulletproof Material: Tungsten has high density and hardness, and is used to manufacture bulletproof materials and armor. Tungsten alloy can provide better bulletproof effect and impact resistance, and is used in the manufacturing of tanks, armored vehicles, and bulletproof vests.
- Medical Field: Tungsten has good biocompatibility and anti solubility, and is widely used in the medical field. Tungsten medical instruments and surgical tools are widely used in surgery and treatment.
- Aerospace Industry: Tungsten has high strength and high-temperature stability, and is widely used in the aerospace industry. Tungsten alloy is used to make engine nozzles for spacecraft and nozzles for rocket engines.
- Energy Sector: With the increasing demand for energy and the development of clean energy, the application of tungsten in the energy sector is gradually increasing. Tungsten alloys are used as battery materials, cladding materials in nuclear reactors, and solar cell modules.
- Automotive Industry: Tungsten alloy is widely used in automotive manufacturing, for manufacturing engine piston rings, gasoline nozzles, brake discs, and transmission components. Tungsten alloys play an important role in the automotive industry due to their excellent high-temperature stability and wear resistance.
- Chemical Industry: Tungsten catalysts have the characteristics of high activity and long lifespan, and are widely used in catalytic reactions in the chemical industry, such as liquid alkane alkylation and dehydrogenation reactions.
Tungsten Properties

Non Ferrous Metals
Tungsten is a non-ferrous metal. Usually, people classify metals into two categories based on their color and properties: ferrous metals and non-ferrous metals. Black metals mainly refer to iron, manganese, chromium and their alloys, such as steel, pig iron, ferroalloys, cast iron, etc. Metals other than ferrous metals are called non-ferrous metals. Tungsten belongs to the category of non-ferrous metals.
The strength and hardness of non-ferrous alloys are generally higher than those of ferrous metals, with higher resistance and lower temperature coefficient of resistance. They have good comprehensive mechanical properties. Therefore, as a non-ferrous metal, tungsten has very high strength and hardness. Due to this characteristic, tungsten carbide with high hardness and strong wear resistance is widely used in cutting tools and mining tools.
Refractory Metal

Tungsten is the refractory metal with the highest melting point. Metals with a melting point higher than 1650 ℃ and a certain reserve, as well as metals with a melting point higher than zirconium (1852 ℃), are generally referred to as refractory metals. Typical refractory metals include tungsten, tantalum, molybdenum, niobium, hafnium, chromium, vanadium, zirconium, and titanium. As a refractory metal, the most important advantage of tungsten is its excellent high-temperature strength and good corrosion resistance to molten alkali metals and vapors. Tungsten only exhibits oxide volatilization and liquid phase oxides above 1000 ℃.
Tungsten also has the disadvantage of having a high temperature for plastic brittle transition, making it difficult to plastic process at room temperature. Refractory metals represented by tungsten have been widely used in industries such as metallurgy, chemical engineering, electronics, light sources, and mechanical industry.
Rare Metals

Tungsten is a rare metal. Rare metals usually refer to metals that are less abundant or sparsely distributed in nature. Tungsten is a widely distributed element that is almost ubiquitous in various types of rocks, but its content is relatively low. The content of tungsten in the crust is 0.001%, and the average content in granite is 1.5 × 10-6, this characteristic makes its extraction very difficult, and usually can only be separated and extracted using organic solvent extraction and ion exchange methods.
With the progress of science and technology, the development of metallurgical processes, equipment, and analysis and detection technology, as well as the expansion of rare metal production scale, the purity and performance of tungsten have been continuously improved, and the variety has been continuously increased, thus expanding the application field of tungsten.
Strategic Metals

Tungsten is a strategic metal. As is well known, rare metals are important strategic resources, and tungsten is a typical rare metal with extremely important uses. It is an important component of contemporary high-tech new materials, and a series of electronic optical materials, special alloys, new functional materials, and organic metal compounds all require the use of tungsten.
Tungsten Types
Tungsten mineral resources are relatively abundant, with a tungsten content of 0.001% in the crust. More than 20 types of tungsten minerals and tungsten containing minerals have been discovered. But among them, only wolframite and scheelite have economic value for mining.

- Wolframite: tungsten manganese ore, wolframite, wolframite;
- Scheelite: scheelite (calcium tungsten ore), molybdenum scheelite, copper scheelite;
- Tungsten Hua Category: water tungsten Hua, high iron tungsten Hua, yttrium tungsten Hua, copper tungsten Hua, water tungsten aluminum ore;
- Uncommon Tungsten Ores: wolframite, clinopyroxenite, molybdenum wolframite, tungsten zinc ore, tungsten bismuth ore, antimony tungsten pyrochlore, titanium yttrium thorium ore (containing tungsten), sulfur tungsten ore, etc.
Although more than 20 types of tungsten minerals and tungsten containing minerals have been discovered, only wolframite and scheelite have economic value for mining, with wolframite accounting for about 30% of the global tungsten resources and scheelite accounting for about 70%.
Tungsten Distribution
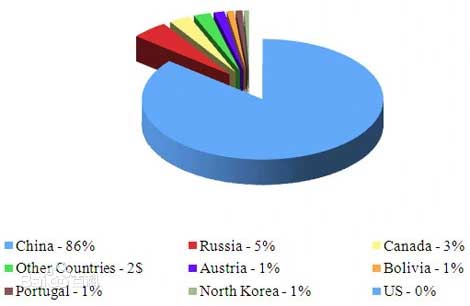
The world tungsten resources are mainly concentrated in the Alps the Himalayas and the circum Pacific geological belt. The tungsten resources in Russia are mainly concentrated in the middle section of the Sikhot Aryn Mountains in the North Caucasus, East Siberia, and Far East regions, with larger mines such as Verkhne Kayrakty. The tungsten resources in the United States are mainly concentrated in California and Colorado. The Canadian tungsten mines are mainly concentrated in the Selwyn tungsten belt, with the most commercially valuable deposits located in the Cassiar batholith in British Columbia to the MacMillan deposit extension at the Yukon and Northwest border.
The global tungsten resource reserves are approximately 3.3 million tons, with China having the largest tungsten resource reserves of 1.9 million tons, accounting for approximately 58% of the global total. Canada (290000 tons), Russia (250000 tons), and the United States (140000 tons). Other countries with abundant tungsten reserves in the world include Bolivia (53000 tons), Australia (10000 tons), and Portugal (4200 tons).
 Flotation Machine | Working | Applications | Types | Select
Flotation Machine | Working | Applications | Types | Select Quartz Sand Applications in 10 Different Industrial Fields
Quartz Sand Applications in 10 Different Industrial Fields limestone | Composition | Properties | Source | 6 Types | 10+ Uses
limestone | Composition | Properties | Source | 6 Types | 10+ Uses Eddy Current Separator & Types | Versus Electrostatic Separation
Eddy Current Separator & Types | Versus Electrostatic Separation



